Pioneering
Next-Generation Therapeutics
At MLS Bio, we are addressing the world's most critical health challenges through AI drug development platforms and innovative small-molecule therapies with our breakthrough compounds. We are targeting a new cellular mode of action, offering novel approaches to treatment in the most impactful global markets.
.avif)
Strategic Focus in Oncology
We are advancing therapeutics for solid Cancers with
Prostate Cancer as our lead indication
Lead Asset
MLS 269, first in class small molecule CD151 inhibitor targeting cancer metastasis

.svg)
Market Opportunity
USD$15.9B addressable prostate cancer therapeutics market


Stage
Proceeding to toxicology, Pre-IND FDA submission


Partnerships
The Prostate Cancer Clinical Trails Consortium
.png)

.svg)
Our
Commitment
Our Global team of experienced medical researchers, commercialization, and technical experts are committed to developing treatments that are more effective, accessible, and better tolerated, delivering novel solutions and improving the lives of patients facing cancer and other life-threatening diseases worldwide.
With our robust pipeline, we aim to develop solutions in other high-impact global markets, bringing transformative therapies to market and delivering better outcomes for patients facing cancer and other life-threatening diseases.
.avif)
.svg)
The Science
CD151, a tetraspanin family protein, is overexpressed in aggressive prostate cancer (PCa), promoting cell migration and invasion, hallmarks of metastasis.
It forms complexes with integrins (e.g., α3β1, α6β1), facilitating tumor cell motility, survival, and vascular invasion.
Experimental inhibition of CD151 results in a significant reduction in cancer cell migration and invasion in vitro and in vivo.
MLS-269 selectively targets the CD151-integrin interface, disrupting critical signaling required for metastatic dissemination.
This host - directed therapeutic approach reduces tumor invasiveness without directly targeting tumor proliferation - highlighting the possibility of limiting drug resistance.
Tetraspanins, particularly CD151, regulate cell motility and promote tumour progression via integrin interactions. Despite their central role in cancer invasion and metastasis, they remain under-explored as therapeutic targets.
Addresses a key driver of cancer progression: metastatic spread.
First-in-class mechanism offers potential for: Survival extension in mCRPC and nmCRPC populations.
Pre-Clinical Validation: MLS 269 reduces prostate cancer tumors, sustained effect over time with body weight neutral or positive profile.
Indication expansion into other CD151-high malignancies (e.g., kidney, lung, pancreatic, breast).
Results suggest that CD151 could be a potential biomarker for prognosis and as a diagnostic in prostate cancer, where higher CD151 levels correlate with reduced survival.
First-in-class therapy innovation targeting cancer metastasis - the leading cause of cancer death - with the potential to significantly extend survival in treatment-resistant prostate and other major solid cancers.
Comprehensive preclinical package supports the application of Phase 1b-2 trials in prostate cancer. A robust translational foundation that de-risks progression to human studies.
Data (in-vivo) supports favourable tolerability when compared to standard of care treatments.
MLS-269 delivers a differentiated anti-metastatic profile in a market dominated by androgen signaling therapies - positioning it for premium pricing, partnership optionality, and potential platform expansion across solid tumors.
Addressing Challenges in
Advanced Cancer Care
Existing therapies provide only partial solutions, with limitations in tolerability and efficacy against resistant, late-stage disease
MLS Bio is transforming patient outcomes by redefining the standard of care in prostate cancer — delivering new hope to patients with advanced disease by expanding treatment options, improving access, and ensuring quality of life through safer, more tolerable therapies
Clinical Significance
Addresses a key driver of prostate cancer progression: metastatic spread.
First-in-class mechanism offers potential for:

Survival extension in mCRPC and nmCRPC populations

Indication expansion into other CD151-high malignancies (e.g., kidney, lung, pancreatic, breast)
First-in-class therapy innovation targeting cancer metastasis - the leading cause of cancer death - with the potential to significantly extend survival in prostate and other major solid cancers.
Preclinical
Validation
Strong In Vivo Efficacy Demonstrated
Our preclinical studies show compelling results:
Conclusion:
MLS Anti-CD151 therapies demonstrate marked tumour suppression, outperforming existing leading therapies and validating CD151 as a promising therapeutic target in metastatic prostate cancer.
.png)
Pipeline
Our Development Portfolio
Leading with Oncology, Expanding Across High-Impact Markets
- Target: First-in-class CD151 tetraspanin inhibitor
- Indication: Metastatic castration-resistant prostate cancer (mCRPC)
- Stage: Proceeding to toxicology, Pre IND-FDA submission & Phase 1b/2 clinical trials.
- Market Opportunity: $15.9B global prostate cancer therapeutics market
- Regulatory Status: Pre IND FDA submission: fast track; breakthrough therapy; priority review
- Target: First in class viral entry inhibitor; in vivo reversing lung damage from infection.
- Indication: Human Health - Broad-spectrum anti-viral, viruses: CMV and Corona Viruses
- Stage: Proceeding to toxicology & Phase 1b/2 Clinical trials.
- Market Opportunity: $85B+ antiviral market by 2030
- Regulatory Status: MLS 289 has PRE–IND FDA approval
- Target: First in class anti-viral agent inhibiting the function of CD181
- Indication: Porcine Reproductive Respiratory Syndrome (PRRS) – all strains
- Impact: Addresses >$2.5B in annual swine industry losses
- Stage: Phase 1/2 large herd trials
- Pathway: Accelerated regulatory pathways (APVMA, FDA-CVM)
- Path to Market with Progressive Market Registrations and combination therapies.
- Clear Platform Expansion Potential
- MLS 269 Expected to be effective in multiple solid tumour cancers
- Oncology Pipeline Comprehensive Expansion:
A successful regulatory and clinical blueprint in prostate cancer de-risks expansion, into other solid tumours, enabling label extensions or new analogues across a high-value platform technology model.
.avif)
Clinical Development
Development Timeline: Clear Path to Clinical Proof-of-Concept, Registration and Exit
Clinical Research Strategy

Toxicology Studies
MLS Bio’s Anti-CD151 therapies have shown strong tolerability in preclinical models, with animals maintaining or gaining weight. This supports high confidence in positive toxicology results, which are expected within 8–10 months and represent a key value-inflection milestone for the company.

Phase 1b/2 Clinical Trials
Evaluate safety, pharmacokinetics & preliminary anti-tumour efficacy in patients with advanced prostate cancer, establishing human data and a recommended dose for Phase 3 trials.
Clinical Partnership
Excellence
Regulatory Advantages
Accelerating Access, Maximizing Value
Our Team
The leadership and advisory at MLS Bio brings together internationally distinguished commercial and scientific expertise encompassing key capabilities for successful pharmaceutical commercialisation. The Board and executive leaders include key experts in financial and legal governance, intellectual property and global regulatory strategy together with commercially experienced professionals who have successfully scaled medical ventures to global exits, directed multi-billion-dollar investment funds, and advanced transformative therapies into international markets. Strengthened by a world-class Scientific Advisory Board—comprising pre-eminent oncologists, endocrinologists, infectious disease specialists, and translational medicine authorities. This combination of scientific rigor, commercial acumen, and governance discipline ensures that MLS Bio is uniquely positioned to advance a portfolio of breakthrough small-molecule therapeutics, driving innovation to patient impact, while delivering sustainable value to investors.
Partners
Building a network to accelerate our mission of MLS Bio has strategically developed a global ecosystem of world-class partners to accelerate the advancement of its pipeline. These strategic collaborations will strengthen the company’s scientific rigor, operational excellence, and international reach. This integrated network positions MLS Bio to accelerate the commercialisation of breakthrough science, bringing transformative therapies to patients and markets worldwide.
Academic





Med-Chem CMC



Drug Formulation


%201.avif)

Preclinical & Clinical





Government and
Industry Supporters





Clinical Excellence Partners

Prostate Cancer Clinical Trials Consortium (PCCTC) - Global clinical trial leadership

Memorial Sloan Kettering Cancer Center Leading cancer center partnership
Professional Services & Strategic Advisors
.svg)
FB Rice
Comprehensive IP strategy development
.svg)
Pace Analytical USA
Expert life-science regulatory partners advising on global regulatory strategy.
Intellectual
Property
Global Patent Portfolio
US & Australian Patents Granted
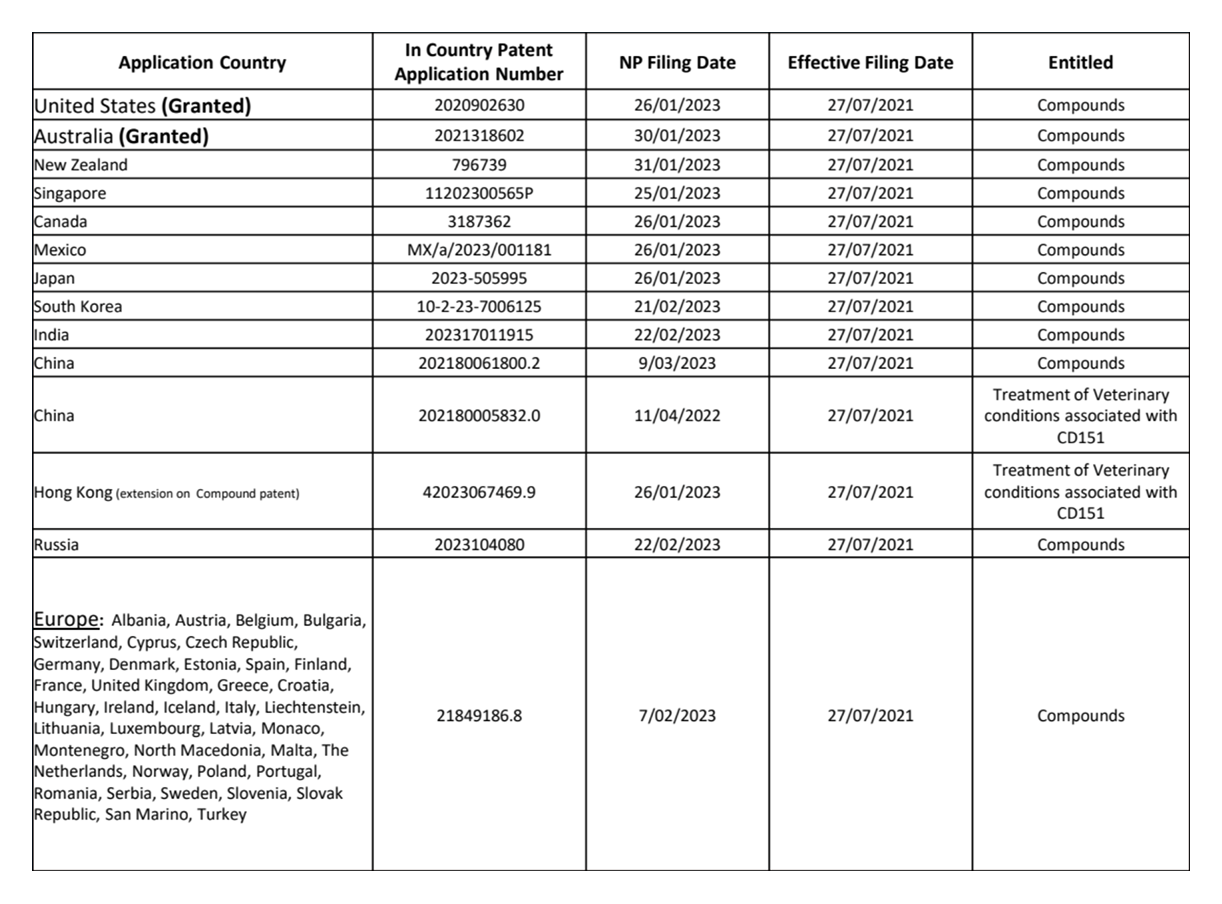
Maximise Patent Life: Regulatory Extensions
U.S. PTEs and European SPCs can extend exclusivity by up to 5 years
MLS-269's patents are active to 2041, an evergreen strategy to add 5–20 years, securing ROI
Strategic Filings and Prosecution: MLS Bio's integrated approach aligns patent prosecutionwith clinical milestones
MLS-269’s globally integrated patent extension strategy is a critical value driver extending revenue lifecycles, mitigating risks, enhancing investor returns, and strengthening positioning for strategic partnerships or acquisitions
Patent Strategy
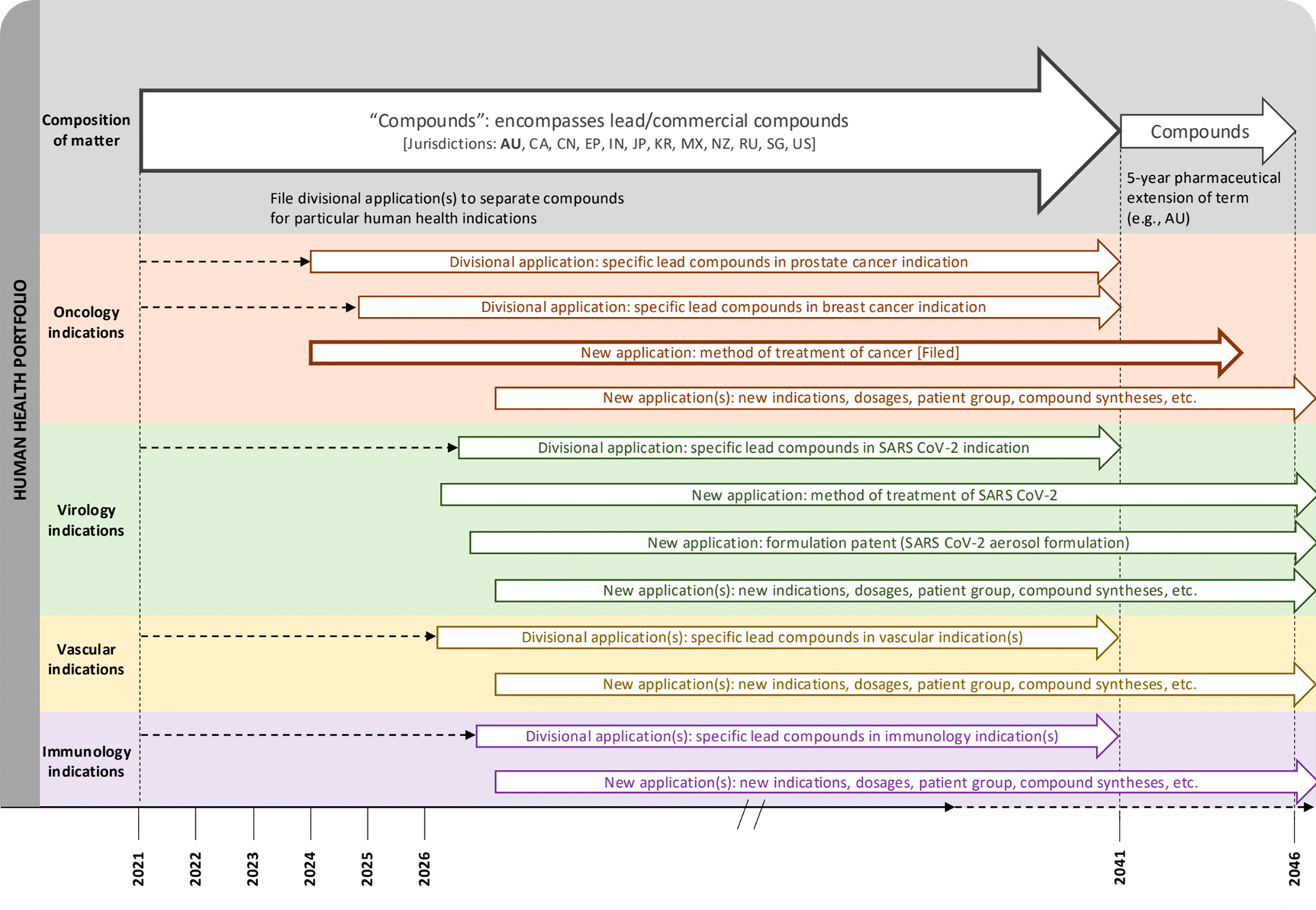 View Patent Strategy
View Patent StrategyInvestment
Thesis
MLS Bio offers investors a de-risked, high-upside opportunity with multiple exit pathways
Investor Insight: MLS Bio’s scientifically unified platform unlocks multi-channel revenue, strategic global partnerships, and high-value exit flexibility, positioning the company as a scalable, multi-sector growth engine that creates:

Multi-channel revenue streams

Cross-sector investment appeal

Optionality for future growth, partnerships, or divestitures
MLS 269 – Prostate Cancer
MLS Bio offers investors a de-risked, high-upside opportunity with multiple exit pathways offering near-term value inflection and long-term upside in a $15 B+ global prostate cancer market.

Differentiated Competitive & Clinical Advantage

Targeting a Significant and Growing Market

Substantial Commercial and Pricing Opportunity

Regulatory and IP Positioning for Longevity

Platform Expansion Potential

Multiple Market Validated Exit Opportunities; through M&A or Strategic Partnerships

News & Updates
“MLS Bio is pioneering small-molecule therapeutics that transform outcomes in prostate cancer, addressing the urgent need for effective options in advanced and resistant disease”
Contact Us
Leadership
Penelope Lane - Managing Director, Chief Executive Officer

penelope@mlsbio.com
Investor Relations
For investment opportunities and corporate updates:

investors@mlsbio.com
Business Development & Partnerships
For strategic partnerships and licensing opportunities:

partnerships@mlsbio.com
Corporate Information
MLS Bio
Level 10, 440 Collins Street Melbourne, Victoria 3000, Australia
Data Room Access
Comprehensive Data Room Available
Due Diligence scientific analysis, market information and financials available under CDA with suitable investor alignment.



.png)
.png)
%20(1).png)